Learn how VHHs can revolutionize CAR-T cell therapy
Small, stable and robust, VHHs offer an attractive construct for use as targeting agents in cell therapies.
Explore the benefits of VHHs in CAR-T cell therapy and learn how this innovative approach can enhance the effectiveness of next-generation cancer treatments.
Advantages of
VHH in Cell Therapy

Smaller constructs mean more space for multi-specific targeting modules or next-generation signalling domains.
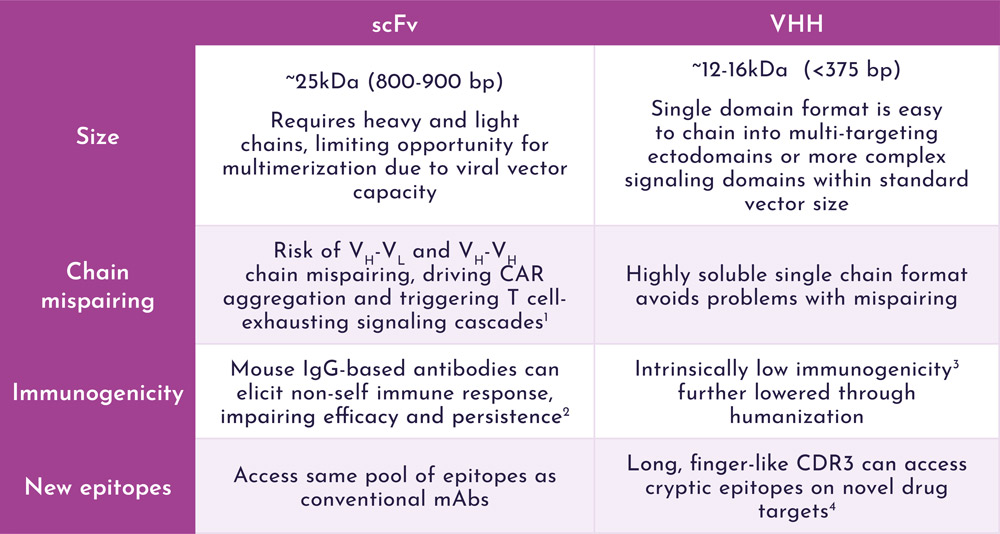
References:
1. Jayaraman J et al. (2020) Ebiomedicine 58: 102931
2. Lamers CH, et al. (2011) Blood 117(1):72-82
3. Ackaert C et al. (2021) Front. Immunol. 12: 632687
4. Desmyter A et al. (1996) Nat Struct Biol 3(9): 803-811

Isogenica’s
VHH in CAR-T Resources
Review: Immunogenicity Risk Profile of Nanobodies
Ackaert et al review the immunogenicity risk profile of two VHHs from Phase II clinical trials, examining pre-existing ADAs and comparing to 3 months post-administration.
Review: The Application of Nanobody in CAR-T Therapy
Bao et al review the intersection between CAR-T development and VHHs against over a dozen tumour-associated antigens, with a focus on the bi-specific VHH therapy LCAR-B38M, now approved as CARVYKTI™.
VHHs enable multi-specific targeting for Tandem-CAR therapies
In this poster from 7th CAR-TCR Summit in Boston, we discuss the power of VHHs in creating multi-specific ectodomains.
Partnerships at Isogenica
Isogenica’s partnerships with global biopharmaceutical companies have resulted in a deep pipeline including one clinical Phase II asset and eleven partnered pre-clinical and discovery programs.


